Species with the same structure: Polyglycol p-750 (dow) Propylene Glycol; … k° H = Henry’s law constant for solubility in water at 298.15 K … propylene glycol and 1,3-butanediol, Nippon Kagaku. Kaishi, 1972, 1972, 1733-1776. Parks and Huffman, 1927 Parks, G.S.; Huffman, H.M., Studies on glass. I. The
Ethylene Glycol – Molecule of the Month – June 2018 (HTML version)
Propylene glycol, or 1,2-propanediol, is a stable, colorless, odorless, viscous liquid with a specific gravity of 1.036. It is an excellent solvent because it is freely miscible with water yet dissolves hydrophobic substances. Among the glycols, propylene glycol has the lowest toxicity.1 Unlike ethylene glycol, propylene glycol does not cause

Source Image: numerade.com
Download Image
Apr 4, 2022Instant Answer: Step 1/3 1. Propylene glycol (C3H8O2) is a polar molecule and will dissolve in water to form propylene glycol molecules and water molecules. It does not ionize, so the major species present will be propylene glycol molecules and water molecules. Step 2/3 2.

Source Image: link.springer.com
Download Image
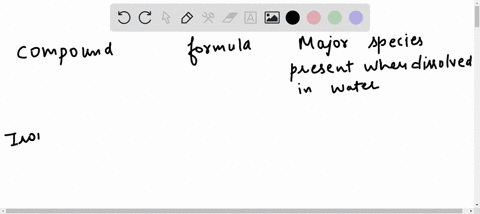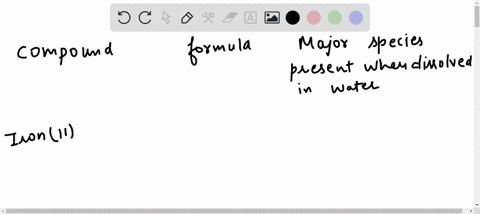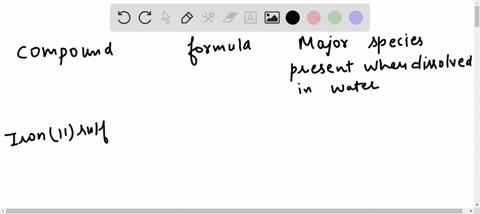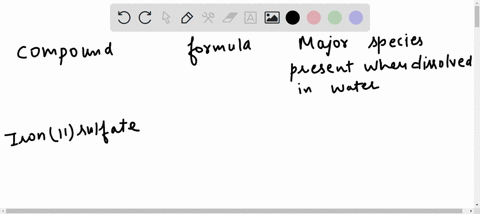
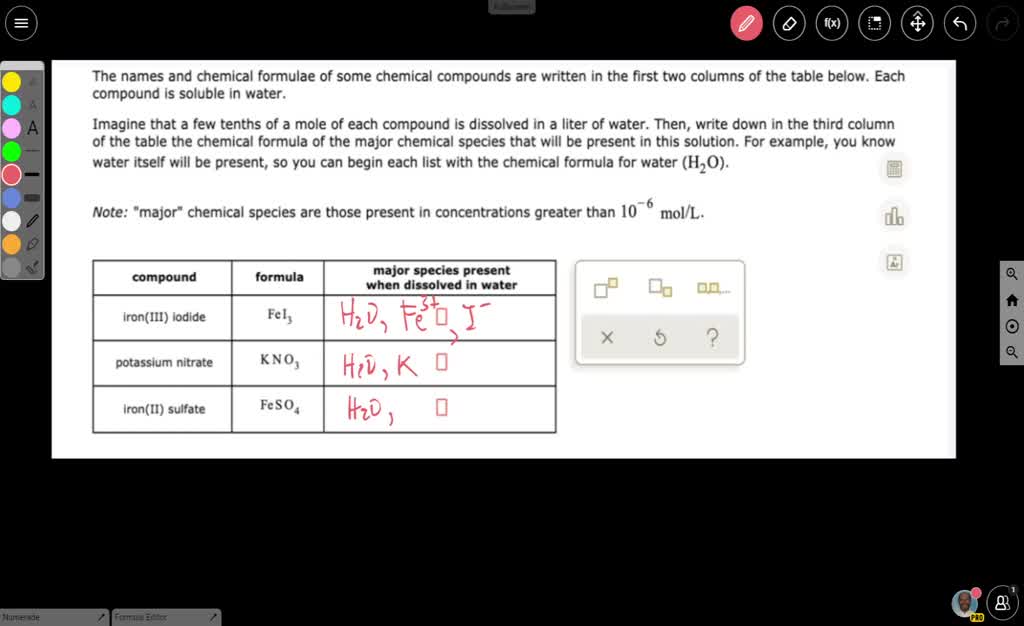
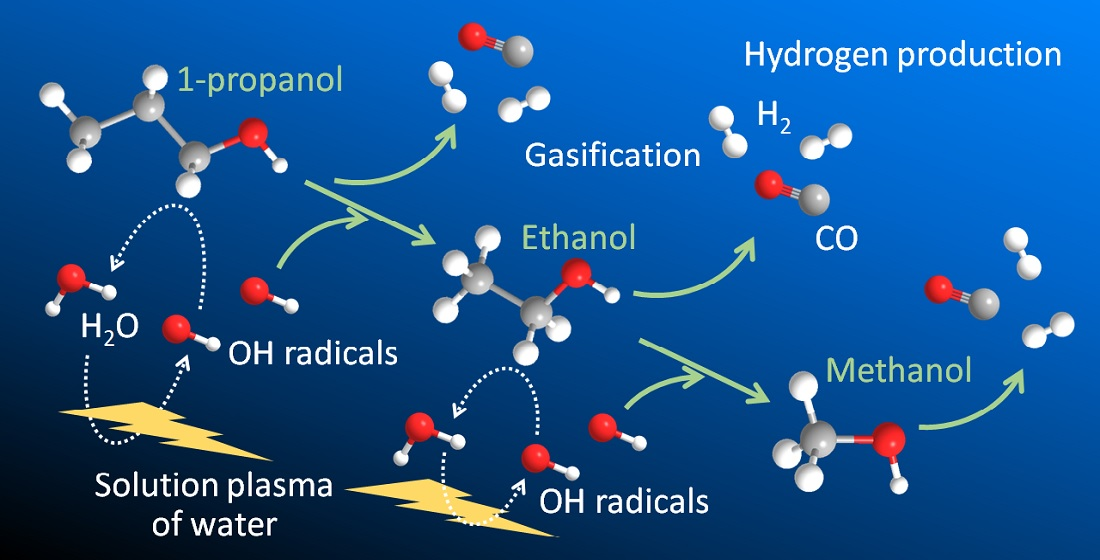
Hydrogen | Free Full-Text | Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: “major” chemical species are those present in concentrations greater than 10 9- mol/L. major species present when dissolved in water compound formula O,0,. propylene glycol C;H, (OH), methanol CH, OH nickel(II) bromide

Source Image: pubs.acs.org
Download Image
Major Species Present When Dissolved In Water Propylene Glycol
For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: “major” chemical species are those present in concentrations greater than 10 9- mol/L. major species present when dissolved in water compound formula O,0,. propylene glycol C;H, (OH), methanol CH, OH nickel(II) bromide Propylene Glycol The propylene glycol substances comprise a homologous family of synthetic organic molecules that have widespread use and very high production volumes across the globe. The information presented and summarized here is intended to provide an overview of the most current and reliable information availa …
Thermodynamic Modeling and Solubility Measurement of Cetirizine Hydrochloride and Deferiprone in Pure Solvents of Acetonitrile, Ethanol, Acetic Acid, Sulfolane, and Ethyl Acetate and Their Mixtures | Journal of Chemical & Engineering Data
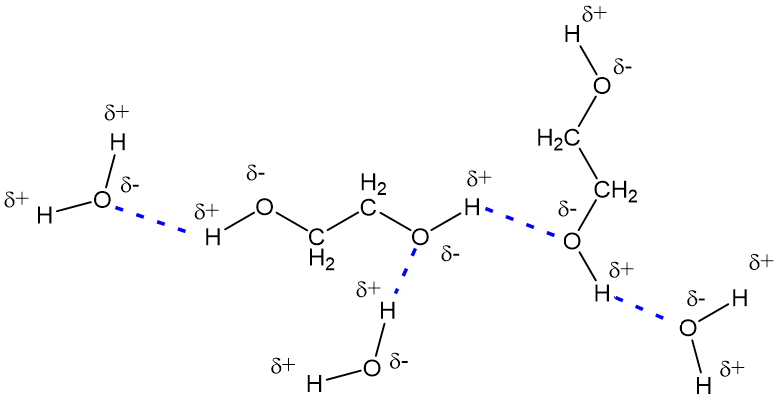
That’s why he mixes with water in every ratio. He can make hydrogen bonds through 2 donors (two H in alcohol groups) and 4 acceptors (2 lone electron pairs each from every O in the molecule). So yes, C X 3 H X 6 (O H) X 2 \ceC_3H_6(OH)_2 C X 3 H X 6 (OH) X 2 is the major species found when dissolving propylene glycol in water. SOLVED: The names and chemical formulas of some chemical compounds are written in the Imagine that a few tenths of a mole of each compound is dissolved in a liter of water. The major chemical species that will be present in this solution. For example …
Source Image: numerade.com
Download Image
SOLVED: The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound soluble in water Imagine that a few tenths of That’s why he mixes with water in every ratio. He can make hydrogen bonds through 2 donors (two H in alcohol groups) and 4 acceptors (2 lone electron pairs each from every O in the molecule). So yes, C X 3 H X 6 (O H) X 2 \ceC_3H_6(OH)_2 C X 3 H X 6 (OH) X 2 is the major species found when dissolving propylene glycol in water.
Source Image: numerade.com
Download Image
Ethylene Glycol – Molecule of the Month – June 2018 (HTML version) Species with the same structure: Polyglycol p-750 (dow) Propylene Glycol; … k° H = Henry’s law constant for solubility in water at 298.15 K … propylene glycol and 1,3-butanediol, Nippon Kagaku. Kaishi, 1972, 1972, 1733-1776. Parks and Huffman, 1927 Parks, G.S.; Huffman, H.M., Studies on glass. I. The
Source Image: chm.bris.ac.uk
Download Image
Hydrogen | Free Full-Text | Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism Apr 4, 2022Instant Answer: Step 1/3 1. Propylene glycol (C3H8O2) is a polar molecule and will dissolve in water to form propylene glycol molecules and water molecules. It does not ionize, so the major species present will be propylene glycol molecules and water molecules. Step 2/3 2.
Source Image: mdpi.com
Download Image
Solved] Note: “major” chemical species are those present in… | Course Hero Feb 24, 2023In conclusion, the major species present when propylene glycol, silver nitrate, and zinc chloride are dissolved in water are the individual atoms of carbon, hydrogen, and oxygen for propylene glycol, the silver ion and nitrate ion for silver nitrate, and the zinc ion and chloride ions for zinc chloride.
Source Image: coursehero.com
Download Image
Insights on production mechanism and industrial applications of renewable propylene glycol – ScienceDirect For example, you know water itself will be present, so you can begin each list with the chemical formula for water (H,O). Note: “major” chemical species are those present in concentrations greater than 10 9- mol/L. major species present when dissolved in water compound formula O,0,. propylene glycol C;H, (OH), methanol CH, OH nickel(II) bromide

Source Image: sciencedirect.com
Download Image
Propylene Glycol – Technical Library Propylene Glycol The propylene glycol substances comprise a homologous family of synthetic organic molecules that have widespread use and very high production volumes across the globe. The information presented and summarized here is intended to provide an overview of the most current and reliable information availa …
Source Image: hydratech.co.uk
Download Image
SOLVED: The names and chemical formulae of some chemical compounds are written in the first two columns of the table below. Each compound soluble in water Imagine that a few tenths of
Propylene Glycol – Technical Library Propylene glycol, or 1,2-propanediol, is a stable, colorless, odorless, viscous liquid with a specific gravity of 1.036. It is an excellent solvent because it is freely miscible with water yet dissolves hydrophobic substances. Among the glycols, propylene glycol has the lowest toxicity.1 Unlike ethylene glycol, propylene glycol does not cause
Hydrogen | Free Full-Text | Gasification of Lower Monohydric Alcohols by Solution Plasma Treatment and Its Reaction Mechanism Insights on production mechanism and industrial applications of renewable propylene glycol – ScienceDirect Feb 24, 2023In conclusion, the major species present when propylene glycol, silver nitrate, and zinc chloride are dissolved in water are the individual atoms of carbon, hydrogen, and oxygen for propylene glycol, the silver ion and nitrate ion for silver nitrate, and the zinc ion and chloride ions for zinc chloride.