Jul 28, 2023The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here: Note that the sum of the formal charges in each case is equal to the charge of the ion (-1).
CHEM 1411 Chapter 6 Homework Flashcards | Quizlet
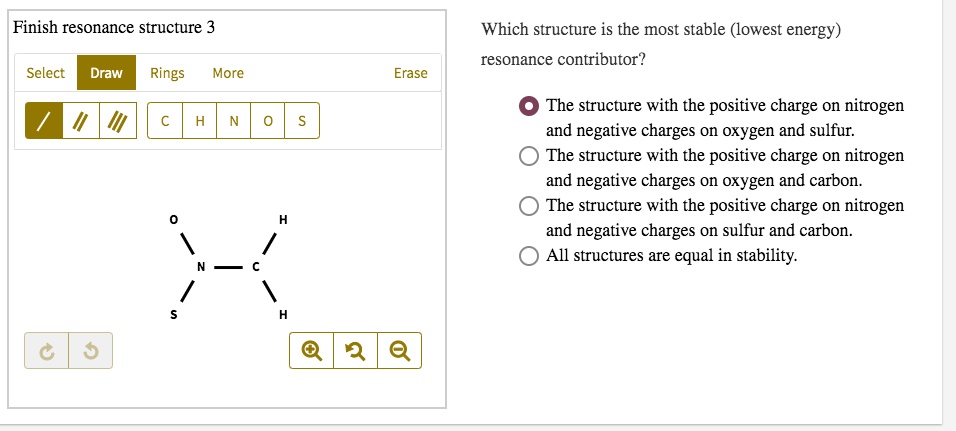
Concept explainers Question Transcribed Image Text: Add formal charges to each resonance form of HCNO. Resonance structure A Resonance structure B н — н — с Resonance structure C Based on the formal charges you added, which structure is favored? A В H -i=N =ö C :ö: :u: Expert Solution Trending now This is a popular solution! Step by step
Source Image: numerade.com
Download Image
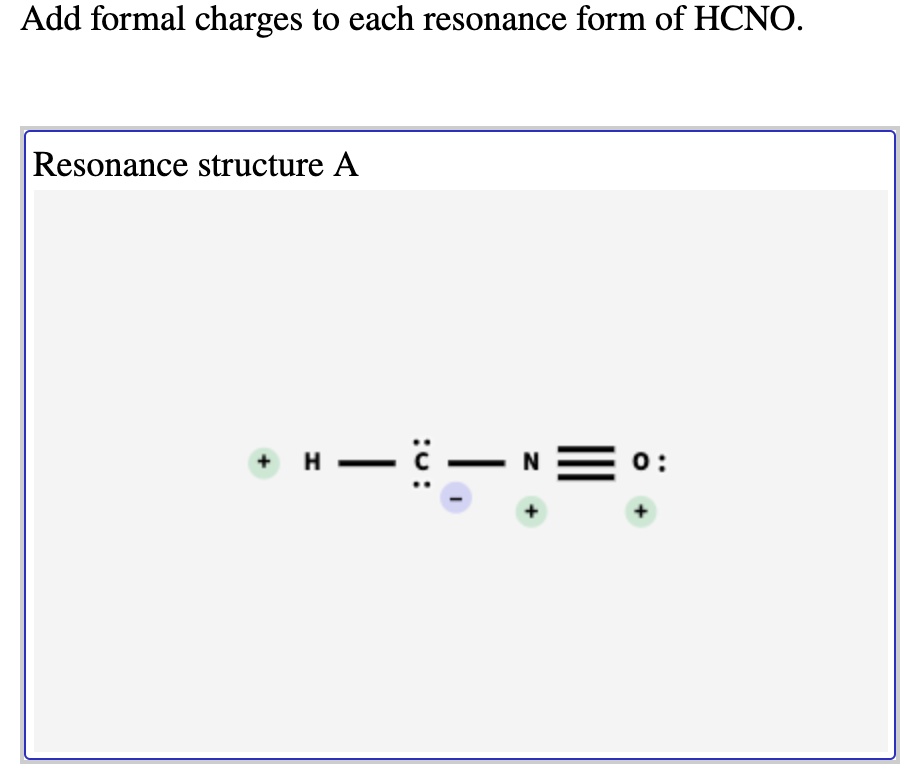
1. A 2. B 3. C Assigning formal charge The formal charge of each atom in a Lewis dot structure is (1/2 the number of electrons in its bonds) + (the total of its electrons in lone pairs)

Source Image: pubs.acs.org
Download Image
SOLVED: Add formal charges to each resonance form of HCNO. Resonance structure A H C I Incorrect Science Add formal charges to each resonance form of HCNO below. Based on the formal charges you added… Question: Add formal charges to each resonance form of HCNO
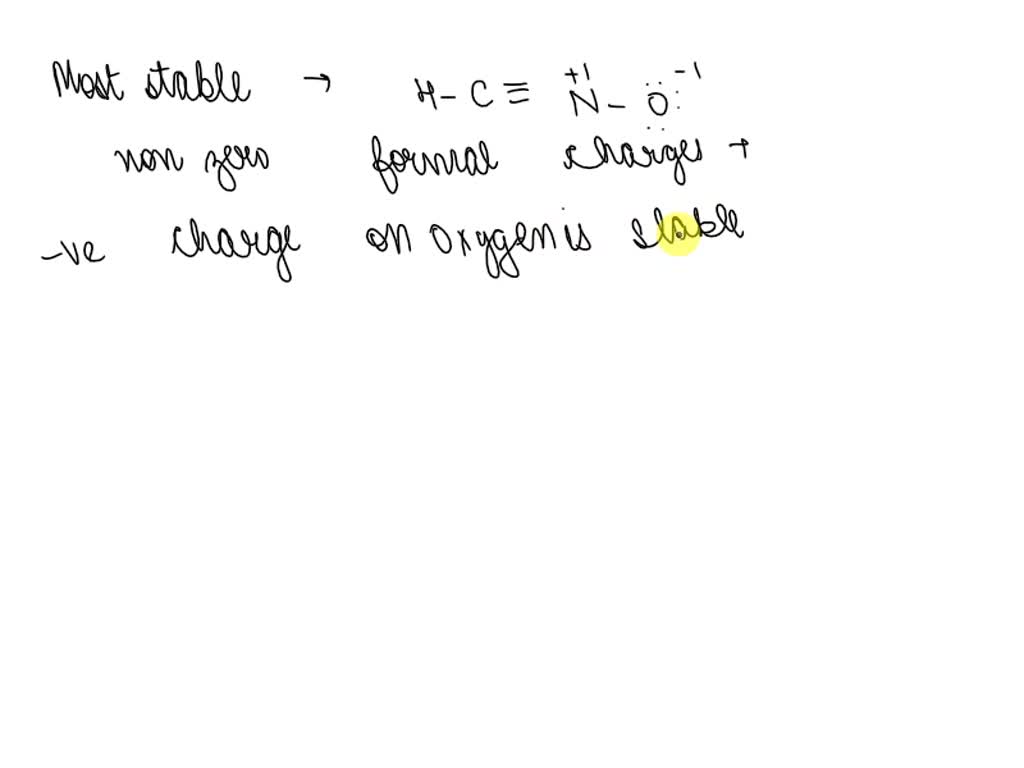
Source Image: numerade.com
Download Image
Add Formal Charges To Each Resonance Form Of Hcno
Science Add formal charges to each resonance form of HCNO below. Based on the formal charges you added… Question: Add formal charges to each resonance form of HCNO Step 1 Explanation: In given questions gives the 3 resonance structure of HCNO. Find the formula changes and favored str… View the full answer Unlock Unlock Answer Unlock Unlock Previous question Next question Transcribed image text: Add formal charges to each resonance form of HCNO below.
SOLVED: (a) Draw the major resonance form of fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized, and it should include
Add formal charges to each resonance form of HCNO. Resonance structure A Resonance structure C Based on the formal charges you added, which structure Select Draw Templates More / II III C N O H is favored? H=∗C N≡O: structure C Based on the formal charges you adde is favored? A H c¨¨= N = o¨ Not the question you’re looking for? SOLVED: Draw the least important resonance contributor for fulminic acid, HCNO, with the atoms connected as indicated in the formula. Your structure should have nonzero formal charges minimized and should include all
Source Image: numerade.com
Download Image
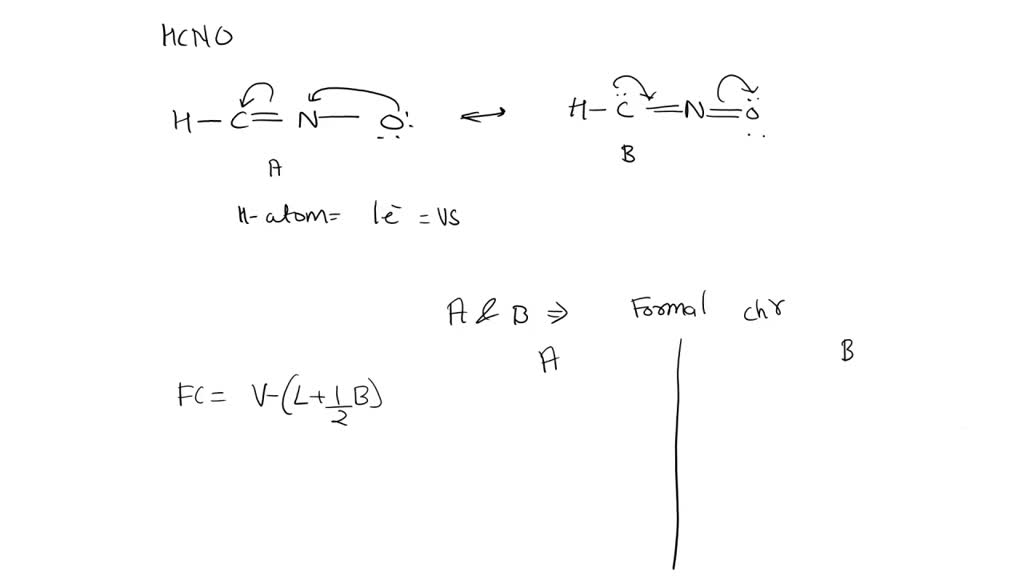
SOLVED: Add formal charges to each resonance form of HCNO. Resonance structure A Hn= 0 : Add formal charges to each resonance form of HCNO. Resonance structure A Resonance structure C Based on the formal charges you added, which structure Select Draw Templates More / II III C N O H is favored? H=∗C N≡O: structure C Based on the formal charges you adde is favored? A H c¨¨= N = o¨ Not the question you’re looking for?
Source Image: numerade.com
Download Image
CHEM 1411 Chapter 6 Homework Flashcards | Quizlet Jul 28, 2023The formal charges present in each of these molecular structures can help us pick the most likely arrangement of atoms. Possible Lewis structures and the formal charges for each of the three possible structures for the thiocyanate ion are shown here: Note that the sum of the formal charges in each case is equal to the charge of the ion (-1).

Source Image: quizlet.com
Download Image
SOLVED: Add formal charges to each resonance form of HCNO. Resonance structure A H C I Incorrect 1. A 2. B 3. C Assigning formal charge The formal charge of each atom in a Lewis dot structure is (1/2 the number of electrons in its bonds) + (the total of its electrons in lone pairs)
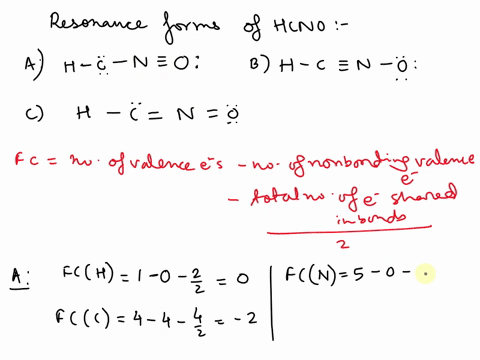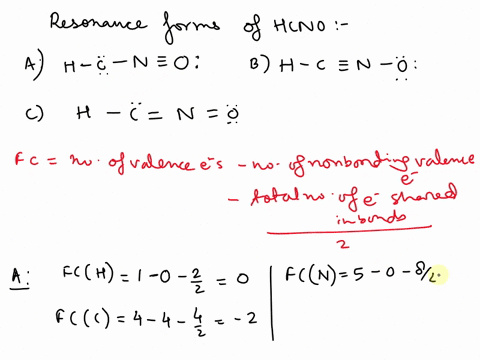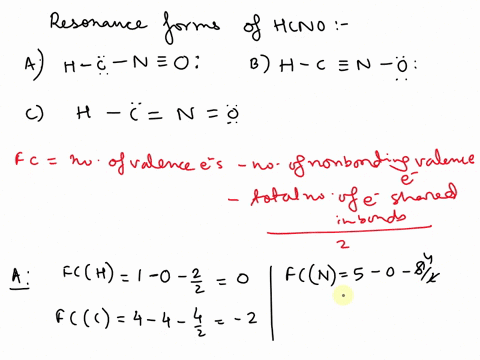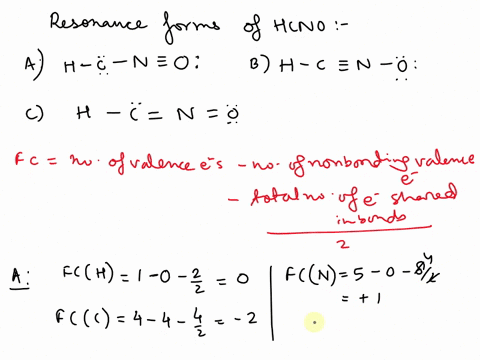
Source Image: numerade.com
Download Image
Rational Design of Persistent Phosphorus-Centered Singlet Tetraradicals and Their Use in Small-Molecule Activation | Journal of the American Chemical Society Aug 13, 2023Add formal charges to each resonance form of HCNO. (Feedback The number of valence electrons in each free atom is H = 1,C =4 N = 5,and 0 = 6. The number of nonbonding electrons on an atom is equal t0 the number of dots around the atom: The number of bonding electrons is equal to twice the number of bonds t0 the atom Resonance structure A

Source Image: pubs.acs.org
Download Image
Solved Add formal charges to each resonance form of HCNO. | Chegg.com Science Add formal charges to each resonance form of HCNO below. Based on the formal charges you added… Question: Add formal charges to each resonance form of HCNO
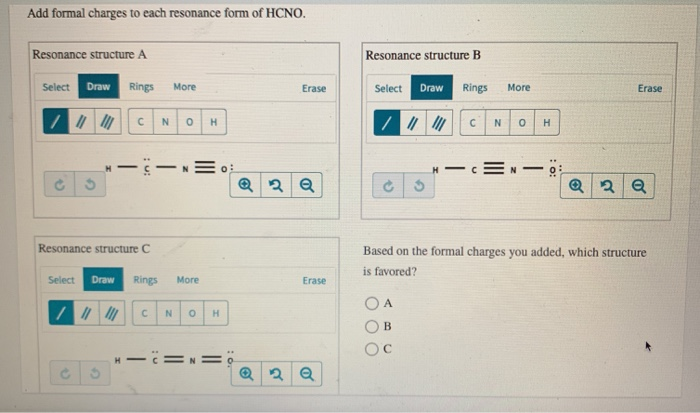
Source Image: chegg.com
Download Image
Solved Add formal charges to each resonance form of HCNO. | Chegg.com Step 1 Explanation: In given questions gives the 3 resonance structure of HCNO. Find the formula changes and favored str… View the full answer Unlock Unlock Answer Unlock Unlock Previous question Next question Transcribed image text: Add formal charges to each resonance form of HCNO below.
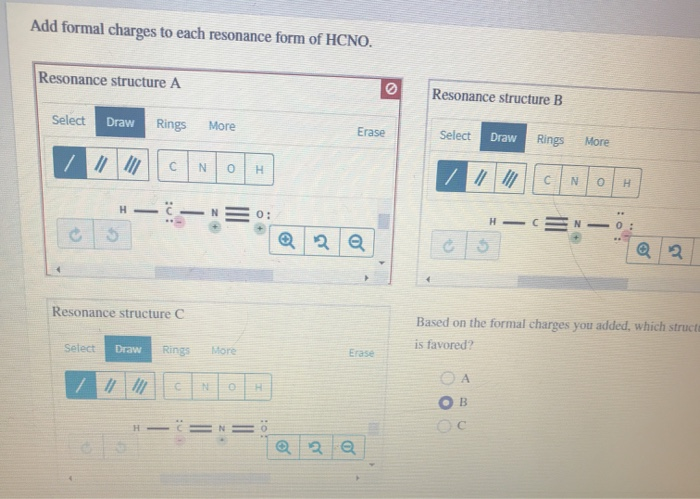
Source Image: chegg.com
Download Image
SOLVED: Add formal charges to each resonance form of HCNO. Resonance structure A Hn= 0 :
Solved Add formal charges to each resonance form of HCNO. | Chegg.com Concept explainers Question Transcribed Image Text: Add formal charges to each resonance form of HCNO. Resonance structure A Resonance structure B н — н — с Resonance structure C Based on the formal charges you added, which structure is favored? A В H -i=N =ö C :ö: :u: Expert Solution Trending now This is a popular solution! Step by step
SOLVED: Add formal charges to each resonance form of HCNO. Resonance structure A H C I Incorrect Solved Add formal charges to each resonance form of HCNO. | Chegg.com Aug 13, 2023Add formal charges to each resonance form of HCNO. (Feedback The number of valence electrons in each free atom is H = 1,C =4 N = 5,and 0 = 6. The number of nonbonding electrons on an atom is equal t0 the number of dots around the atom: The number of bonding electrons is equal to twice the number of bonds t0 the atom Resonance structure A