An atom of hydrogen (atomic number 1) has one proton and one electron. The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. Therefore, the electron configuration of hydrogen is written: For helium (atomic number 2), which has two electrons, the electron configuration is: He: 1s 2
How to Calculate the Energy Absorbed by Electrons Jumping Energy Levels | Physics | Study.com
Share Share The number of sub-levels in each level is equal tothe quantum number: n1. … View the full answer Previous question Next question Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved How many energy sublevels are contained in each of | Chegg.com
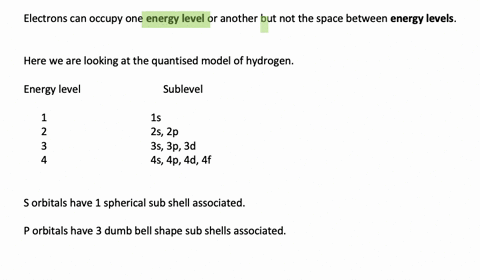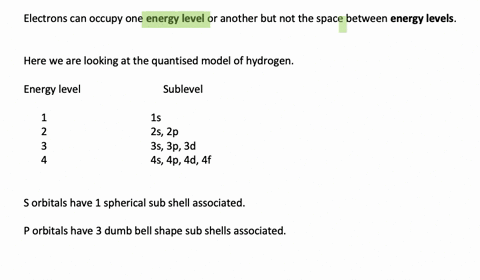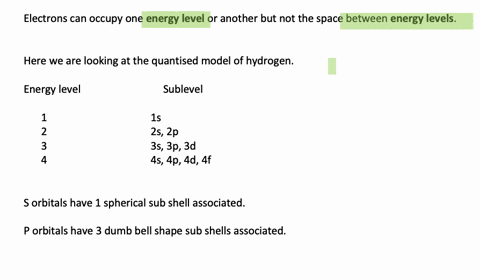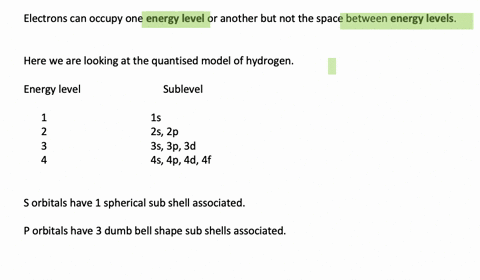
Source Image: www.numerade.com
Download Image
Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Video Answer Solved by verified expert Lottie A.

Source Image: www.pinterest.com
Download Image
Calculate the Energy Levels (En) In a Hydrogen Atom 001 – YouTube Solved stepby-step Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Submitted by Jessica W. Oct. 12, 2021 03:13 p.m. Video Answer Solved by verified expert Video by Lottie Adams University of Manchester | Answered on 09/06/2020 More Than Just

Source Image: study.com
Download Image
Enumerate The Sublevels Contained In The Hydrogen
Solved stepby-step Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Submitted by Jessica W. Oct. 12, 2021 03:13 p.m. Video Answer Solved by verified expert Video by Lottie Adams University of Manchester | Answered on 09/06/2020 More Than Just Figure 7.2.1 7.2. 1: Relationship between the Effective Nuclear Charge Zeff and the Atomic Number Z for the Outer Electrons of the Elements of the First Three Rows of the Periodic Table. Except for hydrogen, Zeff is always less than Z, and Zeff increases from left to right as you go across a row.
Electron Configuration | Overview, Levels & Patterns Video
Sep 20, 2022Each occupied sublevel designation is written followed by a superscript that is the number of electrons in that sublevel. For example, the hydrogen configuration is \(1s^1\), while the helium configuration is \(1s^2\). Multiple occupied sublevels are written one after another. The electron configuration of lithium is \(1s^2 2s^1\). ⏩SOLVED:How many energy sublevels are contained in each of the… | Numerade

Source Image: www.numerade.com
Download Image
9.2 Quantum Theory and The Atom | PDF | Atomic Orbital | Atoms Sep 20, 2022Each occupied sublevel designation is written followed by a superscript that is the number of electrons in that sublevel. For example, the hydrogen configuration is \(1s^1\), while the helium configuration is \(1s^2\). Multiple occupied sublevels are written one after another. The electron configuration of lithium is \(1s^2 2s^1\).

Source Image: www.scribd.com
Download Image
How to Calculate the Energy Absorbed by Electrons Jumping Energy Levels | Physics | Study.com An atom of hydrogen (atomic number 1) has one proton and one electron. The single electron is assigned to the 1s sublevel, the lowest-energy sublevel in the lowest-energy level. Therefore, the electron configuration of hydrogen is written: For helium (atomic number 2), which has two electrons, the electron configuration is: He: 1s 2

Source Image: study.com
Download Image
Calculate the Energy Levels (En) In a Hydrogen Atom 001 – YouTube Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Video Answer Solved by verified expert Lottie A.

Source Image: www.youtube.com
Download Image
11.7 The Hydrogen Orbitals – ChemistrySAANguyen Principle energy levels are color coded, while sublevels are grouped together, and each circle represents an orbital capable of holding two electrons. The lowest energy sublevel is always the \(1s\) sublevel, which consists of one orbital. The single electron of the hydrogen atom will occupy the \(1s\) orbital when the atom is in its ground state.
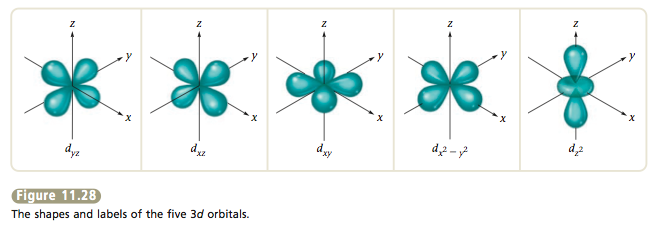
Source Image: chemistrysaanguyen.weebly.com
Download Image
Subshells and Orbitals | PDF | Electron Configuration | Energy Level Solved stepby-step Enumerate the sublevels contained in the hydrogen atom’s first four energy levels. What orbitals are related to each s sublevel and each p sublevel? Submitted by Jessica W. Oct. 12, 2021 03:13 p.m. Video Answer Solved by verified expert Video by Lottie Adams University of Manchester | Answered on 09/06/2020 More Than Just

Source Image: www.scribd.com
Download Image
9.2 Quantum Theory and The Atom | PDF | Atomic Orbital | Atoms Figure 7.2.1 7.2. 1: Relationship between the Effective Nuclear Charge Zeff and the Atomic Number Z for the Outer Electrons of the Elements of the First Three Rows of the Periodic Table. Except for hydrogen, Zeff is always less than Z, and Zeff increases from left to right as you go across a row.

Source Image: www.scribd.com
Download Image
9.2 Quantum Theory and The Atom | PDF | Atomic Orbital | Atoms
9.2 Quantum Theory and The Atom | PDF | Atomic Orbital | Atoms Share Share The number of sub-levels in each level is equal tothe quantum number: n1. … View the full answer Previous question Next question Not the question you’re looking for? Post any question and get expert help quickly. Start learning Answer to Solved How many energy sublevels are contained in each of | Chegg.com
Calculate the Energy Levels (En) In a Hydrogen Atom 001 – YouTube Subshells and Orbitals | PDF | Electron Configuration | Energy Level Principle energy levels are color coded, while sublevels are grouped together, and each circle represents an orbital capable of holding two electrons. The lowest energy sublevel is always the \(1s\) sublevel, which consists of one orbital. The single electron of the hydrogen atom will occupy the \(1s\) orbital when the atom is in its ground state.