Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (Figure 3.6.3 3.6. 3 ). Figure 3.6.3 3.6. 3: Table salt, NaCl, contains an array of sodium and chloride ions combined in a 1:1 ratio.
Pipe Flow vs Pressure – Relationship & Calculate Tools
Molar Mass of Ammonia NH 3 – Step 1: The first step for calculating molar mass is to identify all the elements in a given molecule and write their atomic masses using the periodic table. The atomic mass is equal to the atomic number which is listed below the element symbol. For example, if we are trying calculate for ammonia (NH 3 ), then we

Source Image: researchgate.net
Download Image
Jan 18, 2024μ = m/n The SI unit of molar mass is kg/mol, but the g/mol unit is more commonly used. Molar mass vs. molecular weight It may seem as though the two quantities mean the same thing, but this is not true. Molecular weight and molar mass are different quantities, although numerically equal:
Source Image: thirdspacelearning.com
Download Image
Stoichiometric Calculations: Identify a compound using gravimetric analysis | Labster Virtual Labs
Calculating Molar Mass. Molar mass is the mass of a given substance divided by the amount of that substance, measured in g/mol. For example, the atomic mass of titanium is 47.88 amu or 47.88 g/mol. In 47.88 grams of titanium, there is one mole, or 6.022 x 10 23 titanium atoms.

Source Image: slideserve.com
Download Image
Arrange The Equation To Calculate The Molar Mass M
Calculating Molar Mass. Molar mass is the mass of a given substance divided by the amount of that substance, measured in g/mol. For example, the atomic mass of titanium is 47.88 amu or 47.88 g/mol. In 47.88 grams of titanium, there is one mole, or 6.022 x 10 23 titanium atoms.
The Molar Mass of Molecules. To calculate the molar mass of a molecule, we add the molar mass of each constituent atom by the corresponding subscript. For example, the molar mass of water would be: M (H 2 O) = 2 x 1.0 + 16.0 = 18.0 g/mol. If the formula of the molecule is not given, you will need to first determine it.
PPT – Calculating Molar Mass PowerPoint Presentation, free download – ID:5014630
Dec 5, 20231 Understand molar mass. Molar mass is the mass (in grams) of one mole of a substance. [3] Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element. 2 Find the relative atomic mass of the element.
Calculating molar mass of an oxidant by iodometry – ECHEMI

Source Image: echemi.com
Download Image
How To Calculate The Molar Mass of a Compound – Quick & Easy! – YouTube
Dec 5, 20231 Understand molar mass. Molar mass is the mass (in grams) of one mole of a substance. [3] Using the atomic mass of an element and multiplying it by the conversion factor grams per mole (g/mol), you can calculate the molar mass of that element. 2 Find the relative atomic mass of the element.

Source Image: m.youtube.com
Download Image
Pipe Flow vs Pressure – Relationship & Calculate Tools
Sodium chloride is an ionic compound composed of sodium cations, Na +, and chloride anions, Cl −, combined in a 1:1 ratio. The formula mass for this compound is computed as 58.44 amu (Figure 3.6.3 3.6. 3 ). Figure 3.6.3 3.6. 3: Table salt, NaCl, contains an array of sodium and chloride ions combined in a 1:1 ratio.
Source Image: sino-inst.com
Download Image
Stoichiometric Calculations: Identify a compound using gravimetric analysis | Labster Virtual Labs
Jan 18, 2024μ = m/n The SI unit of molar mass is kg/mol, but the g/mol unit is more commonly used. Molar mass vs. molecular weight It may seem as though the two quantities mean the same thing, but this is not true. Molecular weight and molar mass are different quantities, although numerically equal:
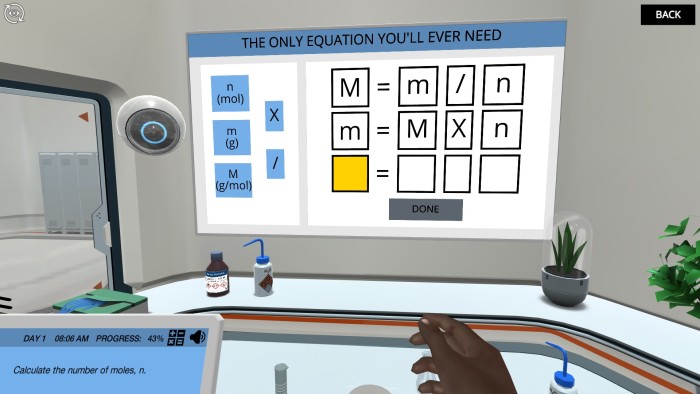
Source Image: labster.com
Download Image
Pharma Engineering: [HOW TO] Calculate Density of solvent Mixture
1. Molar masses of chemical compounds are equal to the sums of the molar masses of all the atoms in one molecule of that compound. If we have a chemical compound like NaCl, the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. If we write this as a calculation, it looks like this:
![Pharma Engineering: [HOW TO] Calculate Density of solvent Mixture](https://2.bp.blogspot.com/-IMT9YlNZa-M/V3ROrtEm7UI/AAAAAAAAAH0/vcCuiz3jQ0MyQfgXYyrDb2TfMC7SARykACLcB/s1600/slide_2.jpg)
Source Image: pharmacalculations.com
Download Image
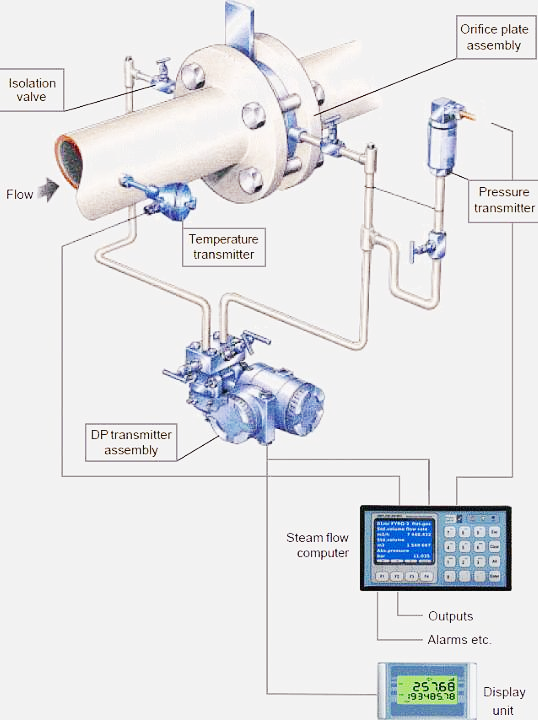
Mass Flow Meter: What is it? How It Works, Types, Accuracy
Calculating Molar Mass. Molar mass is the mass of a given substance divided by the amount of that substance, measured in g/mol. For example, the atomic mass of titanium is 47.88 amu or 47.88 g/mol. In 47.88 grams of titanium, there is one mole, or 6.022 x 10 23 titanium atoms.

Source Image: iqsdirectory.com
Download Image
Answered: Calculate the molar mass of a… | bartleby
The Molar Mass of Molecules. To calculate the molar mass of a molecule, we add the molar mass of each constituent atom by the corresponding subscript. For example, the molar mass of water would be: M (H 2 O) = 2 x 1.0 + 16.0 = 18.0 g/mol. If the formula of the molecule is not given, you will need to first determine it.
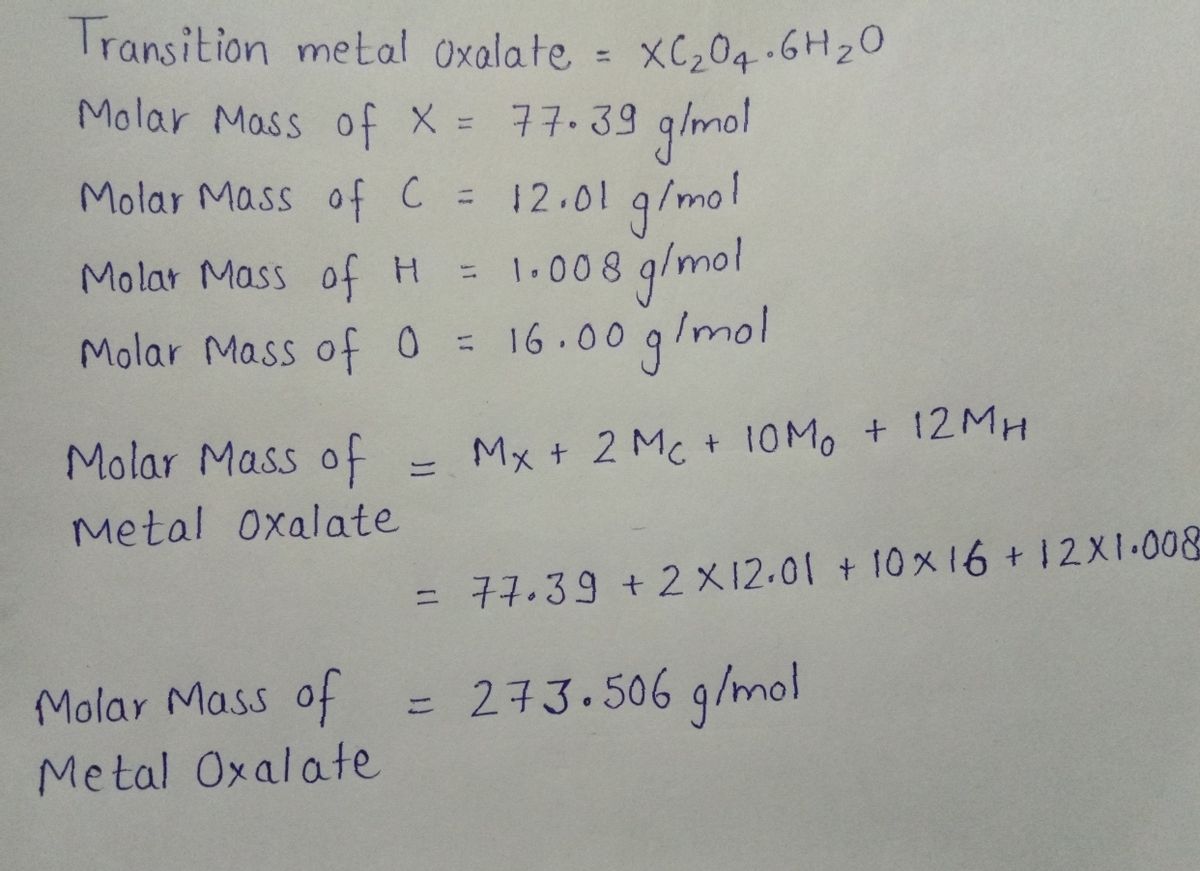
Source Image: bartleby.com
Download Image
How To Calculate The Molar Mass of a Compound – Quick & Easy! – YouTube
Answered: Calculate the molar mass of a… | bartleby
Molar Mass of Ammonia NH 3 – Step 1: The first step for calculating molar mass is to identify all the elements in a given molecule and write their atomic masses using the periodic table. The atomic mass is equal to the atomic number which is listed below the element symbol. For example, if we are trying calculate for ammonia (NH 3 ), then we
Stoichiometric Calculations: Identify a compound using gravimetric analysis | Labster Virtual Labs Mass Flow Meter: What is it? How It Works, Types, Accuracy
1. Molar masses of chemical compounds are equal to the sums of the molar masses of all the atoms in one molecule of that compound. If we have a chemical compound like NaCl, the molar mass will be equal to the molar mass of one atom of sodium plus the molar mass of one atom of chlorine. If we write this as a calculation, it looks like this: