Find step-by-step Chemistry solutions and your answer to the following textbook question: Classify each of the following processes as spontaneous or non-spontaneous: a) the combustion of natural gas b) a hot drink cooling to room temperature c) energy from the ocean’s surface to power a ship d) the extraction of iron metal from iron ore.
SOLVED: Classify each process as spontaneous or nonspontaneous. Spontaneous: Water is separated into H2 and O2 gas at 25°C. A cup of hot coffee cools to room temperature. Perfume molecules spread out
1. Which of the following processes are spontaneous? Click the card to flip 👆 Spontaneous – (a process that occurs on its own without – any outside assistance) alignment of iron filings in a magnetic field – the dissolution of HCl\ ( (g)\) in water to form concentrated hydrochloric acid

Source Image: slideplayer.com
Download Image
1. Classify each of the following as spontaneous or nonspontaneous processes: a) the browning of a cut apple slice on a snack tray over time b) the rolling of a ball uphill c) the formation of diamond from the graphite in your pencil d) the melting of ice cubes in a glass of water you are holding 2. State the second law of thermodynamics. 3.
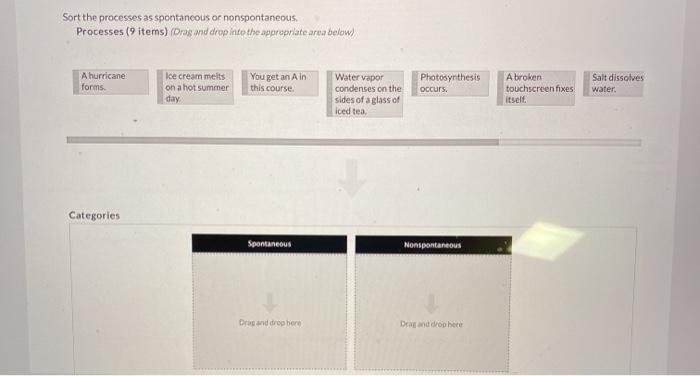
Source Image: chegg.com
Download Image
Determining Spontaneity by using the Gibbs Free Energy Equation | Chemistry | Study.com
Classify each of the following processes as spontaneous or nonspontaneous: (A) combustion of natural gas (B) hot drink cooling to room temperature (C) drawing heat energy from the ocean’s surface to power a ship (D) extraction of iron metal from iron ore Solution Verified Answered 7 months ago Create a free account to view solutions
Source Image: studimed.de
Download Image
Classify Each Of The Following Processes As Spontaneous Or Nonspontaneous.
Classify each of the following processes as spontaneous or nonspontaneous: (A) combustion of natural gas (B) hot drink cooling to room temperature (C) drawing heat energy from the ocean’s surface to power a ship (D) extraction of iron metal from iron ore Solution Verified Answered 7 months ago Create a free account to view solutions
Step 1 Whether a reaction is spontaneous or nonspontaneous depends on the thermodynamics of the reaction, s… View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Classify each of the following processes as spontaneous or non-spontaneous.
Entrance exam materials
Nov 17, 2023A spontaneous process is one that occurs naturally under certain conditions. A nonspontaneous process, on the other hand, will not take place unless it is “driven” by the continual input of energy from an external source. A process that is spontaneous in one direction under a particular set of conditions is nonspontaneous in the reverse
Guide to Chapter 17. Thermodynamics – Mattson

Source Image: yumpu.com
Download Image
Solved Part A Classify the following as spontaneous or | Chegg.com
Nov 17, 2023A spontaneous process is one that occurs naturally under certain conditions. A nonspontaneous process, on the other hand, will not take place unless it is “driven” by the continual input of energy from an external source. A process that is spontaneous in one direction under a particular set of conditions is nonspontaneous in the reverse
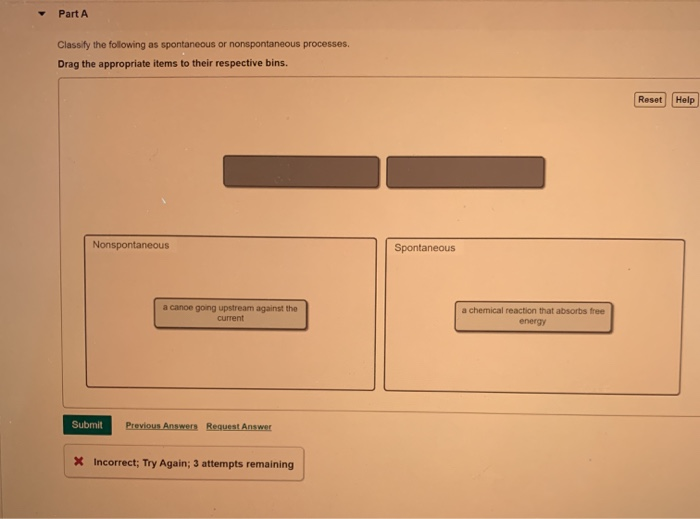
Source Image: chegg.com
Download Image
SOLVED: Classify each process as spontaneous or nonspontaneous. Spontaneous: Water is separated into H2 and O2 gas at 25°C. A cup of hot coffee cools to room temperature. Perfume molecules spread out
Find step-by-step Chemistry solutions and your answer to the following textbook question: Classify each of the following processes as spontaneous or non-spontaneous: a) the combustion of natural gas b) a hot drink cooling to room temperature c) energy from the ocean’s surface to power a ship d) the extraction of iron metal from iron ore.

Source Image: numerade.com
Download Image
Determining Spontaneity by using the Gibbs Free Energy Equation | Chemistry | Study.com
1. Classify each of the following as spontaneous or nonspontaneous processes: a) the browning of a cut apple slice on a snack tray over time b) the rolling of a ball uphill c) the formation of diamond from the graphite in your pencil d) the melting of ice cubes in a glass of water you are holding 2. State the second law of thermodynamics. 3.

Source Image: study.com
Download Image
SOLVED: Classify each of the following processes as spontaneous or nonspontaneous Drag the appropriate processes to their respective bins Reset Help hot drink cooling room temperature drawing heat energy from the oceans
Question: Classify each of the following processes as spontaneous or nonspontaneous. I. H2O (l) → H2O (g) T = 25°C, vessel open to atmosphere with 50% relative humidity II. H2O (s) → H2O (1) T= 25°C, P= 1 atm I is nonspontaneous and II is spontaneous. I and II are both nonspontaneous. I is spontaneous and II is nonspontaneous.
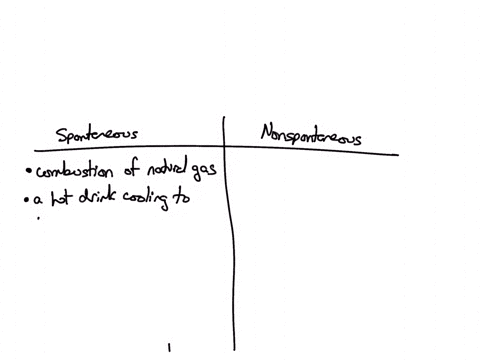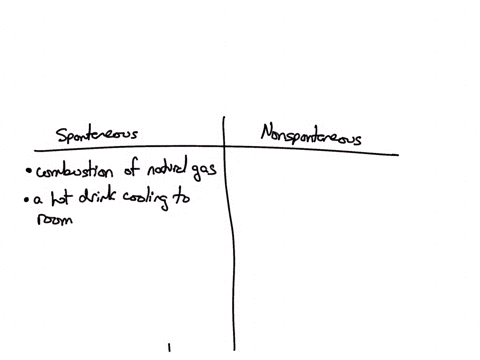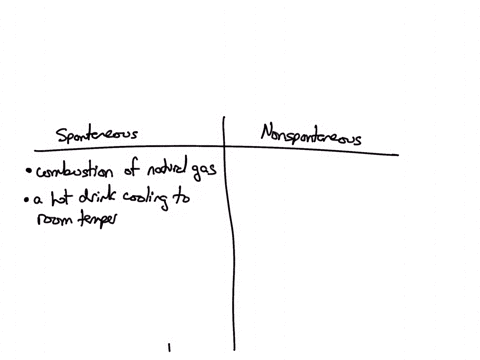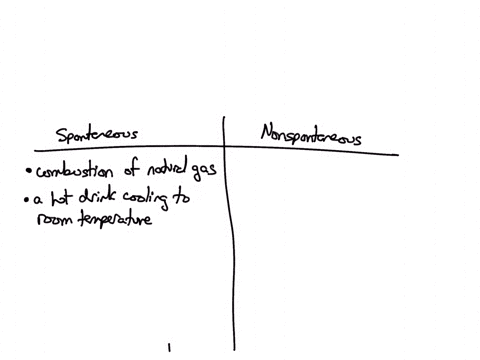
Source Image: numerade.com
Download Image
Spontaneous and Nonspontaneous Processes | Thermodynamics
Classify each of the following processes as spontaneous or nonspontaneous: (A) combustion of natural gas (B) hot drink cooling to room temperature (C) drawing heat energy from the ocean’s surface to power a ship (D) extraction of iron metal from iron ore Solution Verified Answered 7 months ago Create a free account to view solutions

Source Image: nigerianscholars.com
Download Image
Chapter 16: Entropy, Free Energy Equilibrium Flashcards | Quizlet
Step 1 Whether a reaction is spontaneous or nonspontaneous depends on the thermodynamics of the reaction, s… View the full answer Step 2 Unlock Answer Unlock Previous question Next question Transcribed image text: Classify each of the following processes as spontaneous or non-spontaneous.

Source Image: quizlet.com
Download Image
Solved Part A Classify the following as spontaneous or | Chegg.com
Chapter 16: Entropy, Free Energy Equilibrium Flashcards | Quizlet
1. Which of the following processes are spontaneous? Click the card to flip 👆 Spontaneous – (a process that occurs on its own without – any outside assistance) alignment of iron filings in a magnetic field – the dissolution of HCl\ ( (g)\) in water to form concentrated hydrochloric acid
Determining Spontaneity by using the Gibbs Free Energy Equation | Chemistry | Study.com Spontaneous and Nonspontaneous Processes | Thermodynamics
Question: Classify each of the following processes as spontaneous or nonspontaneous. I. H2O (l) → H2O (g) T = 25°C, vessel open to atmosphere with 50% relative humidity II. H2O (s) → H2O (1) T= 25°C, P= 1 atm I is nonspontaneous and II is spontaneous. I and II are both nonspontaneous. I is spontaneous and II is nonspontaneous.